Small Pharma Tackles Major Depression with Potentially Game Changing Psychedelic Therapy
The world is facing a mental health crisis. Today, one in six individuals are likely to suffer from an episode of major depression during their lifeti …

The world is facing a mental health crisis. Today, one in six individuals are likely to suffer from an episode of major depression during their lifetime that could significantly impact their relationships, work and health. Add to that the substantial negative impact the COVID-19 pandemic continues to have, and it’s easy to see how this situation will only get worse.
While treatments exist, about a third of those who suffer from depression never recover. It’s a fact that has spurred efforts to find better therapies.
Small Pharma, a London-based neuropharmaceutical company with a rich history in drug development, thinks it has a potential solution – dimethyltryptamine (DMT).
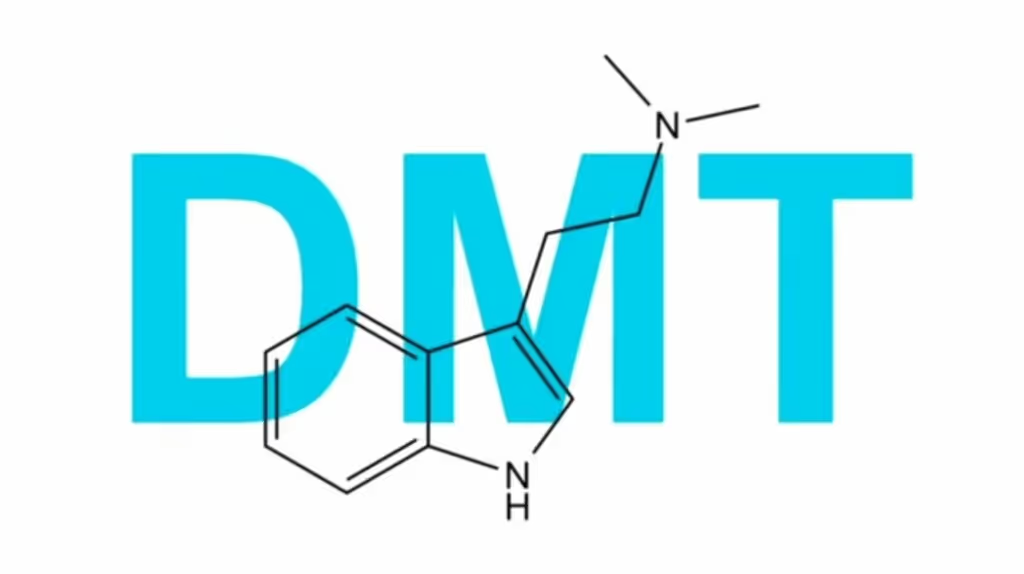
DMT, a naturally occurring molecule found in several plants and animals, is a powerful, short-acting psychedelic believed to have medicinal properties.
The drug is believed to work by significantly increasing the number of electrical connections between brain cells, which in turn may lead to a period of plasticity that is associated with an enhanced receptivity to positive psychological treatment.
Unlike current antidepressant drugs that can take up to 12 weeks to be effective, DMT-based treatments could be almost immediate and longer lasting. They are also targeted at the root causes of depression as opposed to mere symptom suppression, an approach that has people like Peter Rands, CEO and founder of Small Pharma, excited.

“If we get the same kind of results with DMT as we’ve seen with other longer acting psychedelics like psilocybin, we could be looking at a significant improvement over the 30% efficacy rate with SSRIs (Selective Serotonin Reuptake Inhibitors), which are the standard of care in depression treatment at the moment.”
Beyond the therapeutic benefits DMT can potentially offer, there are some potential cost benefits as well.
“DMT produces a short-acting psychedelic experience with a treatment duration that lasts less than thirty minutes, and that really matters,” Rands explains. “One absolutely crucial element to the safety of psychedelic assisted therapy is the setting. That involves health professionals like physicians and therapists whose time will be factored into treatment infrastructure costs. The shorter the experience, the lower those costs are likely to be.”
DMT can also be manufactured cheaply at scale, which Small Pharma hopes will help support their mission to develop novel depression treatments that are widely accessible to all.
Work to validate the use of DMT for psychedelic assisted therapies is well underway. Early in 2021, Small Pharma initiated the world’s first regulated clinical trial for DMT-assisted therapy for major depression, in collaboration with Imperial College London. This past October, the company announced that the first patient had been dosed as part of its Phase IIa clinical trial. Small Pharma is encouraged by the preliminary results from its placebo-controlled Phase I clinical trial involving healthy individuals with no previous psychedelic experience. The study showed that DMT-assisted therapy was found to be well-tolerated and caused no serious side effects to date in the 32 healthy volunteers who participated. The team was able to identify a dose of DMT to take forwards into its Phase IIa clinical trial, where they aim to assess the efficacy, safety, and tolerability of this treatment in patients with Major Depressive Disorder.

In the meantime, Small Pharma is laser-focused on protecting their intellectual property. IP is an essential asset in this industry and Small Pharma has been able to build out an impressive patent portfolio, thanks in large part to Peter Rands, who is a qualified patent attorney in both the UK and Europe. His presence provides Small Pharma with a unique advantage over others in the field like Atai Life Sciences (NASDAQ: ATAI) and Compass Pathways (NASDAQ: CMPS).
To date, Small Pharma has over 30 patents pending in its psychedelic portfolio, some of which cover Composition of Matter protection on a new generation of psychedelic compounds which could offer optimised treatment profiles. In addition, Small Pharma has received a UK grant for one of its patents covering Composition of Matter protection.
With key IP secured, Rands can set his sights towards a current antidepressant market that estimates peg to be about $16 billion[1] [2] . When psychedelic drugs are ultimately approved, their growth potential could be massive.
Small Pharma is getting ready for that day. This year alone, Small Pharma raised $63 million in funding and started trading on the TSX Venture Exchange. According to Rands, “this is expected to provide us the funding to comfortably position ourselves where we can either attract a partner or the follow up equity funding necessary to progress development of this treatment with the hope it reaches patients in health clinics across the world.”
Look out for updates on Small Pharma’s active clinical trial exploring DMT-assisted therapy as a treatment for major depression.
https://www.youtube.com/watch?v=w7-GXX5wQJI

For more information on Small Pharma Inc. (TSX.V: DMT) please click the request investor info button.
FULL DISCLOSURE: Small Pharma is a client of BTV-Business Television. This article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional. Any action taken as a result of reading information here is the reader’s sole responsibility.
Latest Posts
Hot Companies
You might also like