News Alert: FSD Pharma - FDA Approval to Submit I.N.D. Application for Compound to Treat Covid-19 Patients
FSD Pharma is a specialty biotech pharmaceutical company. FSD Pharma BioSciences, Inc., is a wholly-owned subsidiary. Moreover, it is a specialty biot …
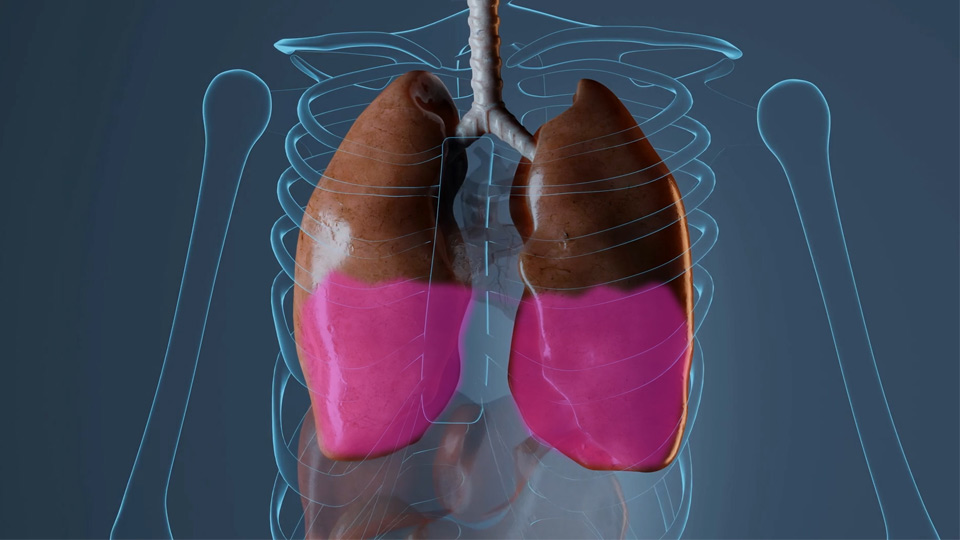
The FDA approved FSD Pharma to submit an Investigational New Drug (I.N.D.) application
FSD Pharma is a specialty biotech pharmaceutical company. FSD Pharma BioSciences, Inc., is a wholly-owned subsidiary. Moreover, it is a specialty biotech pharmaceutical R&D company focused on developing, over time, multiple applications of its lead compound FSD201, ultramicronized palmitoylethanolamide (PEA), by down-regulating the cytokines to effectuate an anti-inflammatory response.
In addition, upon a Phase 1 first-in-human safety study, the compound was deemed safe with no serious adverse side effects. Further, the US Food and Drug Administration recently gave the Company permission to submit an Investigational New Drug application for the use of FSD201 to treat COVID-19.
Severe COVID-19 is characterized by an over-exuberant inflammatory response that may lead to a cytokine storm and ultimately death. Therefore, the Company is focused on developing FSD201 for its anti-inflammatory properties to avoid the cytokine storm associated with acute lung injury in hospitalized COVID-19 patients.
FSD's mission is to focus on the development of FDA approved synthetic compounds that address unmet medical needs.
For more information on FSD Pharma Inc. (CSE: HUGE, NASDAQ: HUGE) please fill out the form below.
Latest Articles
Hot Companies
You might also like

BTV Showcases Contango Ore, Critical Elements Lithium, Dryden Gold, FireFox Gold, Kirkland Lake, Klondike Gold, and Selkirk Mines
This week, BTV - Business Television we feature growth-focused mining companies advancing major projects across North America and Europe. From emerging producers and strategic combinations to high-grade exploration and critical mineral development, this episode highlights the next wave of opportunity in the resource sector, including:

Selkirk Copper: A Yukon restart positioned to deliver new Canadian copper supply by 2028
A 50,000-metre drill program began last summer to expand understanding of the resource and guide updated mine design. The goal is to define a 12–15 year mine life while refreshing permits and engineering.




